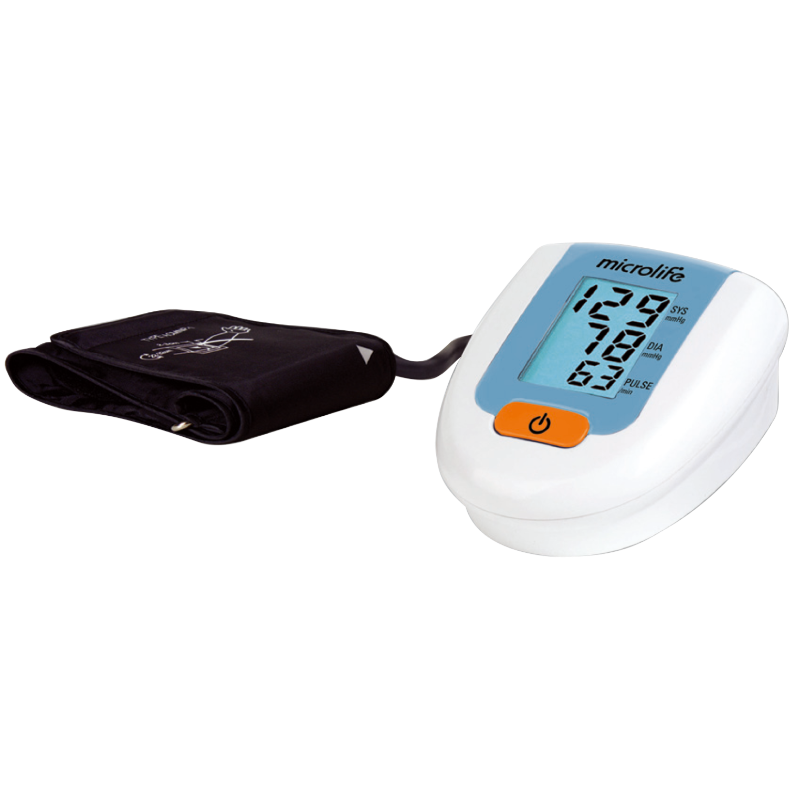
心房顫動提示
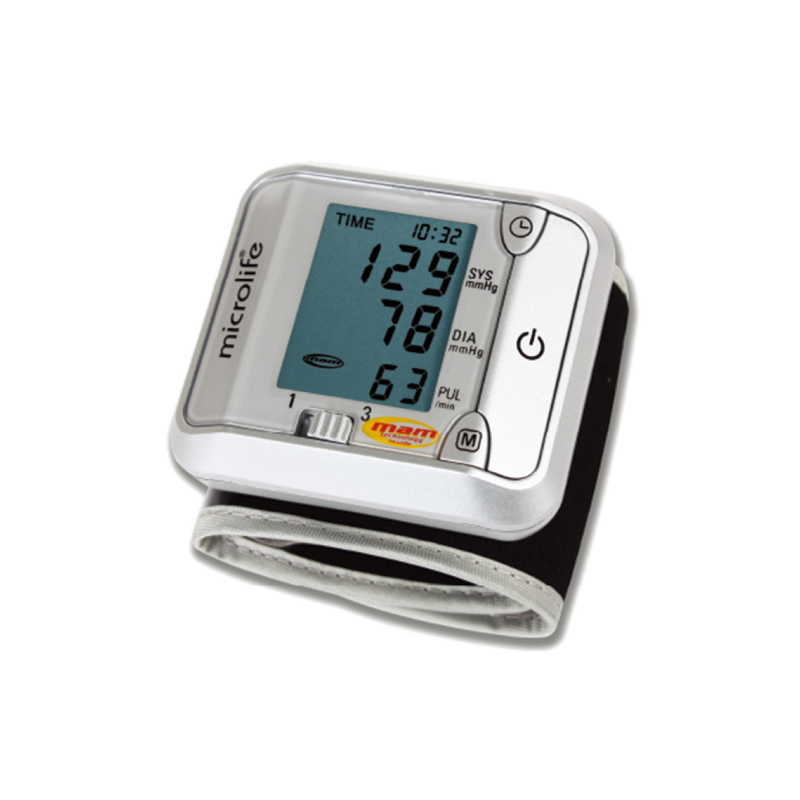
MAM 平均值測量
2 組用戶各99組記憶值
AFIB專利心房顫動偵測技術
MAM智能血壓平均量測技術
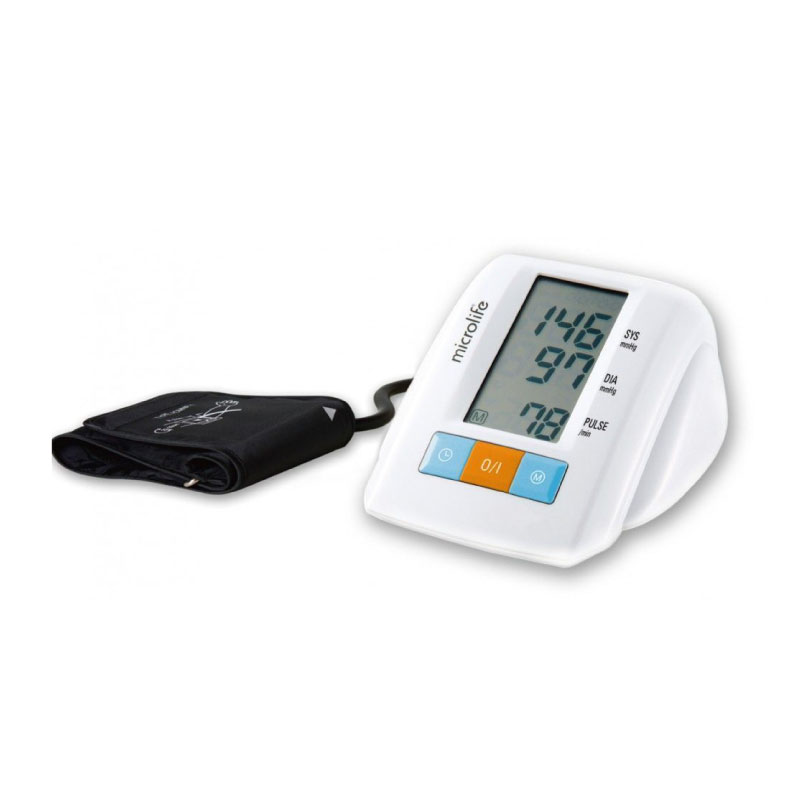
200 組記憶值
AFIB專利心房顫動偵測技術
MAM智能血壓平均量測技術
PAD心跳不規律偵測技術
藍芽傳輸
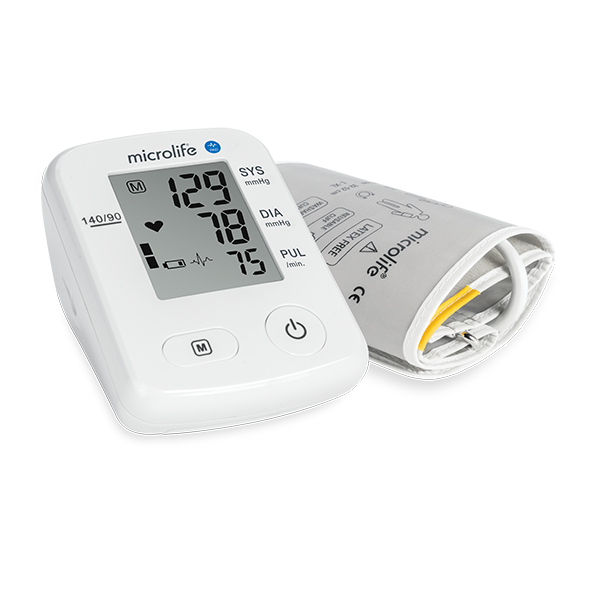
BPA6 BASIC 為Microlife 與歐美頂尖臨床醫師工同合作的心房顫動偵測技術,讓民眾在家就能篩查心房顫動,搭配Microlife 三次平均測量模式,使血壓測量更加精確。
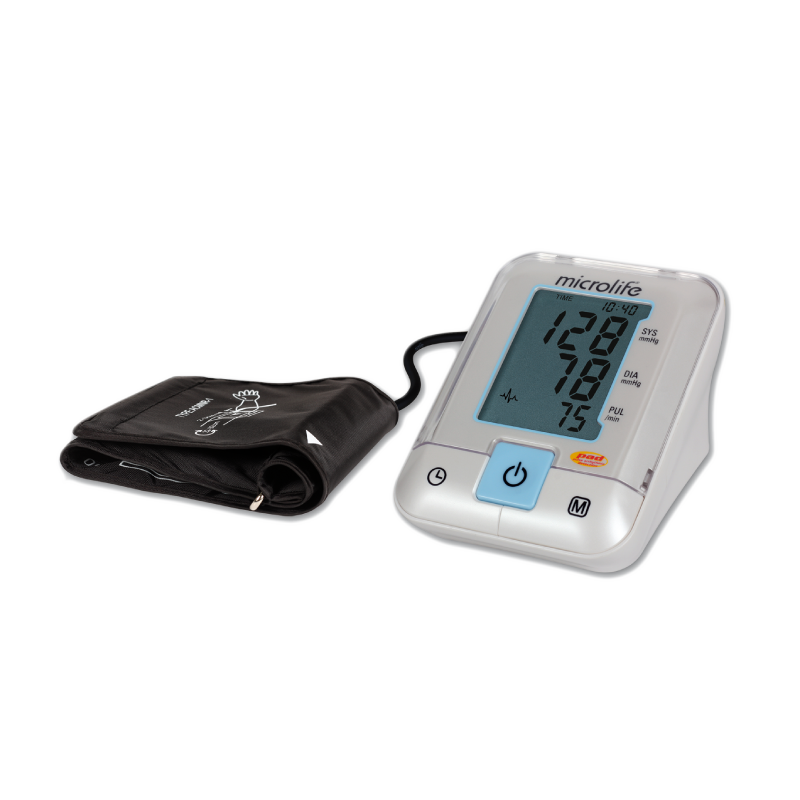
心房顫動提示
MAM 平均值測量
2 組用戶各99組記憶值
高血壓和心房顫動 (AF) 是心臟病和腦中風最常見的可預防的危險因素
https://www.microlife.com.tw/wp-content/uploads/2021/12/3863-BP-W3-Comfort_halfNEW_web.png
600
600
microlife
/wp-content/uploads/2020/09/logo_microlife-3.svg
microlife 2021-12-28 10:23:23 2022-09-13 08:47:16 BP W3 COMFORT 2021 年 12 月 27 日
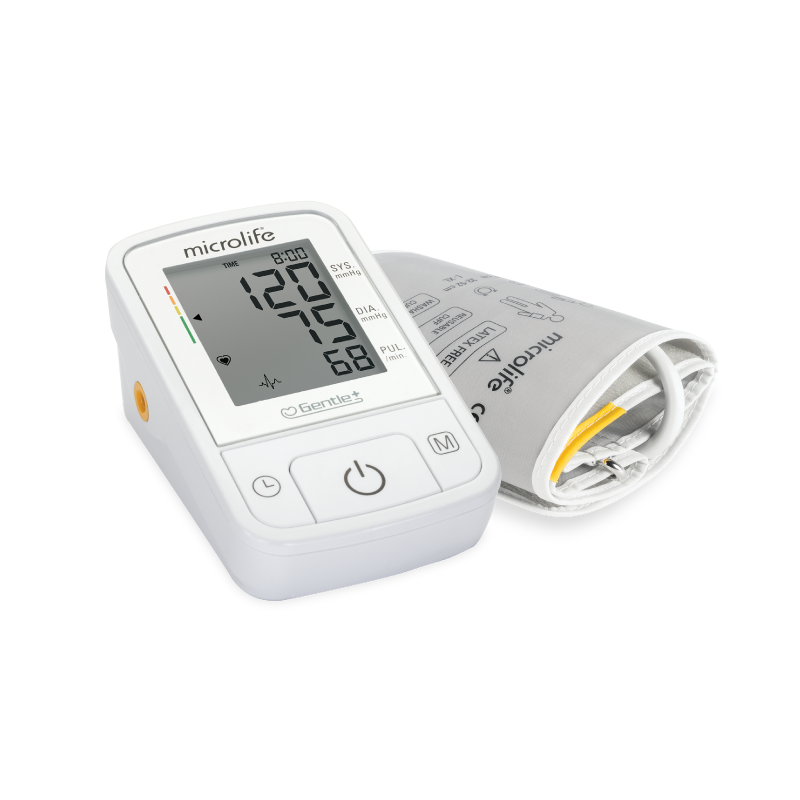
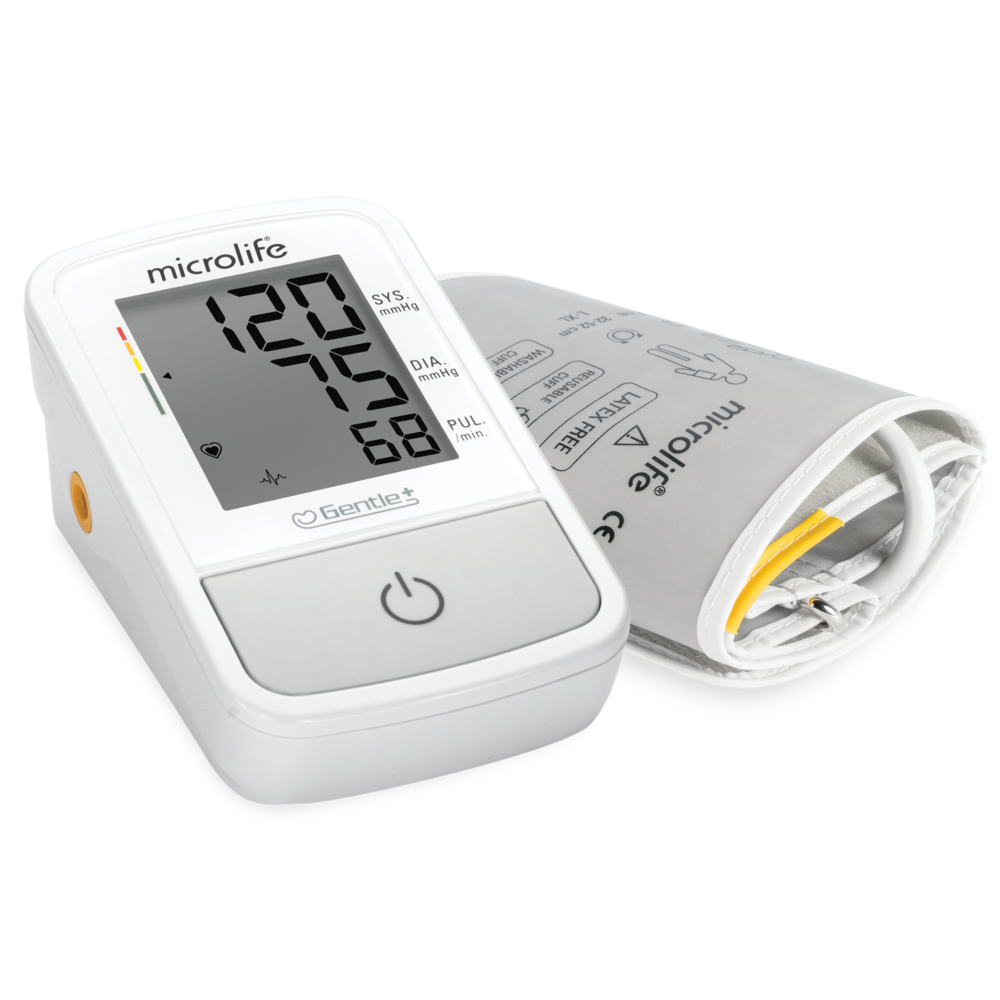
SmartMAM智能平均測量模式
https://www.microlife.com.tw/wp-content/uploads/2021/12/3852-BP-B3-Basic_half_cuff.png
600
600
microlife
/wp-content/uploads/2020/09/logo_microlife-3.svg
microlife 2021-12-27 16:49:19 2022-10-06 11:46:38 BP B3 BASIC 2021 年 12 月 27 日
AFIB專利心房顫動偵測技術
https://www.microlife.com.tw/wp-content/uploads/2021/12/3850-BP-B3-AFIB_half_cuff.png
600
600
microlife
/wp-content/uploads/2020/09/logo_microlife-3.svg
microlife 2021-12-27 16:25:03 2023-03-22 12:00:36 BP B3 AFIB 2021 年 12 月 27 日
PAD心律不整偵測技術
https://www.microlife.com.tw/wp-content/uploads/2021/12/3841-BP-B2-Basic_half-cuff.png
600
600
microlife
/wp-content/uploads/2020/09/logo_microlife-3.svg
microlife 2021-12-27 15:03:31 2023-03-22 12:03:00 BP B2 BASIC 2021 年 12 月 27 日
PAD心律不整偵測技術
https://www.microlife.com.tw/wp-content/uploads/2021/12/BP-A2-Classic_half_cuff_600x600.png
600
600
microlife
/wp-content/uploads/2020/09/logo_microlife-3.svg
microlife 2021-12-27 14:14:44 2022-05-24 13:50:50 BP A2 CLASSIC 2021 年 12 月 27 日
AFIB專利心房顫動偵測技術
https://www.microlife.com.tw/wp-content/uploads/2021/12/BP-A6_BT_MIT-half_cuff_Two-user.png
2000
2000
microlife
/wp-content/uploads/2020/09/logo_microlife-3.svg
microlife 2021-12-27 13:29:10 2022-10-06 11:44:34 BP3GU1-7B 2020 年 9 月 27 日
AFIB專利心房顫動偵測技術
https://www.microlife.com.tw/wp-content/uploads/2020/09/BPA6BT.png
800
800
admin
/wp-content/uploads/2020/09/logo_microlife-3.svg
admin 2020-09-27 20:20:01 2022-10-06 11:45:05 BP A6 BT 2020 年 9 月 27 日
AFIB專利心房顫動偵測技術
https://www.microlife.com.tw/wp-content/uploads/2020/09/BPA6Basic.png
800
800
admin
/wp-content/uploads/2020/09/logo_microlife-3.svg
admin 2020-09-27 20:17:03 2022-05-20 16:40:40 BP A6 BASIC 2020 年 9 月 27 日
MAM智能血壓平均量測技術
https://www.microlife.com.tw/wp-content/uploads/2020/09/BPA3PC.png
800
800
admin
/wp-content/uploads/2020/09/logo_microlife-3.svg
admin 2020-09-27 20:12:37 2022-05-26 09:10:26 BP A3 PC 2020 年 9 月 27 日
MAM智能血壓平均量測技術
https://www.microlife.com.tw/wp-content/uploads/2020/09/BPA3BASIC.png
800
800
admin
/wp-content/uploads/2020/09/logo_microlife-3.svg
admin 2020-09-27 20:07:42 2022-06-01 16:30:04 BP A3 BASIC 2020 年 9 月 27 日
PAD心跳不規律偵測技術
https://www.microlife.com.tw/wp-content/uploads/2020/09/BPA2Basic.png
800
800
admin
/wp-content/uploads/2020/09/logo_microlife-3.svg
admin 2020-09-27 19:51:53 2022-05-24 13:52:19 BP A2 BASIC 2020 年 9 月 27 日
AFIB專利心房顫動偵測技術
https://www.microlife.com.tw/wp-content/uploads/2020/11/BP3MS1-4KT-2.png
800
800
admin
/wp-content/uploads/2020/09/logo_microlife-3.svg
admin 2020-09-27 19:44:24 2022-05-24 14:29:36 BP 3MS1-4KT 2020 年 9 月 27 日
AFIB專利心房顫動偵測技術
https://www.microlife.com.tw/wp-content/uploads/2020/09/BP3MS1-4K-2-.png
800
800
admin
/wp-content/uploads/2020/09/logo_microlife-3.svg
admin 2020-09-27 16:14:50 2022-05-24 14:37:45 BP 3MS1-4K
https://www.microlife.com.tw/wp-content/uploads/2022/09/BP3BU1-3_800.png
800
800
admin
/wp-content/uploads/2020/09/logo_microlife-3.svg
admin 2020-09-27 16:07:28 2022-09-22 10:23:44 BP 3BU1-3
https://www.microlife.com.tw/wp-content/uploads/2022/09/BP3BJ1-4_800.png
800
800
admin
/wp-content/uploads/2020/09/logo_microlife-3.svg
admin 2020-09-27 15:28:56 2022-09-22 17:15:24 BP 3BJ1-4D 2020 年 9 月 26 日
PAD心跳不規律偵測技術
https://www.microlife.com.tw/wp-content/uploads/2020/09/BPA2Easy-e1607993783246.png
1000
1000
admin
/wp-content/uploads/2020/09/logo_microlife-3.svg
admin 2020-09-26 19:58:25 2022-05-24 15:59:16 BP A2 EASY
https://www.microlife.com.tw/wp-content/uploads/2020/09/BP3BG1-A.png
800
800
admin
/wp-content/uploads/2020/09/logo_microlife-3.svg
admin 2020-09-26 15:16:09 2022-05-26 09:14:04 BP 3BG1-A
https://www.microlife.com.tw/wp-content/uploads/2020/09/BP3AQ1-1_square-1.png
800
800
admin
/wp-content/uploads/2020/09/logo_microlife-3.svg
admin 2020-09-26 15:07:49 2022-05-26 10:01:01 BP 3AQ1-1
https://www.microlife.com.tw/wp-content/uploads/2020/09/BP3AQ1-1.jpg
800
800
admin
/wp-content/uploads/2020/09/logo_microlife-3.svg
admin 2020-09-26 14:52:15 2022-05-26 09:59:47 BP 3AQ1
https://www.microlife.com.tw/wp-content/uploads/2020/09/BP3AR1-3P-2.png
800
800
demo
/wp-content/uploads/2020/09/logo_microlife-3.svg
demo 2020-09-26 14:49:47 2022-05-24 16:02:38 BP 3AR1-3P
https://www.microlife.com.tw/wp-content/uploads/2021/05/BP3ML1-A.png
2400
2400
microlife
/wp-content/uploads/2020/09/logo_microlife-3.svg
microlife 2020-06-10 13:54:25 2022-06-07 09:09:00 BP 3ML1-A
https://www.microlife.com.tw/wp-content/uploads/2021/12/3863-BP-W3-Comfort_halfNEW_web.png
600
600
microlife
/wp-content/uploads/2020/09/logo_microlife-3.svg
microlife 2021-12-28 10:23:23 2022-09-13 08:47:16 BP W3 COMFORT 2021 年 12 月 27 日
SmartMAM智能平均測量模式
https://www.microlife.com.tw/wp-content/uploads/2021/12/3852-BP-B3-Basic_half_cuff.png
600
600
microlife
/wp-content/uploads/2020/09/logo_microlife-3.svg
microlife 2021-12-27 16:49:19 2022-10-06 11:46:38 BP B3 BASIC 2021 年 12 月 27 日
AFIB專利心房顫動偵測技術
https://www.microlife.com.tw/wp-content/uploads/2021/12/3850-BP-B3-AFIB_half_cuff.png
600
600
microlife
/wp-content/uploads/2020/09/logo_microlife-3.svg
microlife 2021-12-27 16:25:03 2023-03-22 12:00:36 BP B3 AFIB 2021 年 12 月 27 日
PAD心律不整偵測技術
https://www.microlife.com.tw/wp-content/uploads/2021/12/3841-BP-B2-Basic_half-cuff.png
600
600
microlife
/wp-content/uploads/2020/09/logo_microlife-3.svg
microlife 2021-12-27 15:03:31 2023-03-22 12:03:00 BP B2 BASIC 2021 年 12 月 27 日
PAD心律不整偵測技術
https://www.microlife.com.tw/wp-content/uploads/2021/12/BP-A2-Classic_half_cuff_600x600.png
600
600
microlife
/wp-content/uploads/2020/09/logo_microlife-3.svg
microlife 2021-12-27 14:14:44 2022-05-24 13:50:50 BP A2 CLASSIC 2021 年 12 月 27 日
AFIB專利心房顫動偵測技術
https://www.microlife.com.tw/wp-content/uploads/2021/12/BP-A6_BT_MIT-half_cuff_Two-user.png
2000
2000
microlife
/wp-content/uploads/2020/09/logo_microlife-3.svg
microlife 2021-12-27 13:29:10 2022-10-06 11:44:34 BP3GU1-7B 2020 年 9 月 27 日
AFIB專利心房顫動偵測技術
https://www.microlife.com.tw/wp-content/uploads/2020/09/BPA6BT.png
800
800
admin
/wp-content/uploads/2020/09/logo_microlife-3.svg
admin 2020-09-27 20:20:01 2022-10-06 11:45:05 BP A6 BT 2020 年 9 月 27 日
AFIB專利心房顫動偵測技術
https://www.microlife.com.tw/wp-content/uploads/2020/09/BPA6Basic.png
800
800
admin
/wp-content/uploads/2020/09/logo_microlife-3.svg
admin 2020-09-27 20:17:03 2022-05-20 16:40:40 BP A6 BASIC 2020 年 9 月 27 日
MAM智能血壓平均量測技術
https://www.microlife.com.tw/wp-content/uploads/2020/09/BPA3PC.png
800
800
admin
/wp-content/uploads/2020/09/logo_microlife-3.svg
admin 2020-09-27 20:12:37 2022-05-26 09:10:26 BP A3 PC 2020 年 9 月 27 日
MAM智能血壓平均量測技術
https://www.microlife.com.tw/wp-content/uploads/2020/09/BPA3BASIC.png
800
800
admin
/wp-content/uploads/2020/09/logo_microlife-3.svg
admin 2020-09-27 20:07:42 2022-06-01 16:30:04 BP A3 BASIC 2020 年 9 月 27 日
PAD心跳不規律偵測技術
https://www.microlife.com.tw/wp-content/uploads/2020/09/BPA2Basic.png
800
800
admin
/wp-content/uploads/2020/09/logo_microlife-3.svg
admin 2020-09-27 19:51:53 2022-05-24 13:52:19 BP A2 BASIC 2020 年 9 月 27 日
AFIB專利心房顫動偵測技術
https://www.microlife.com.tw/wp-content/uploads/2020/11/BP3MS1-4KT-2.png
800
800
admin
/wp-content/uploads/2020/09/logo_microlife-3.svg
admin 2020-09-27 19:44:24 2022-05-24 14:29:36 BP 3MS1-4KT 2020 年 9 月 27 日
AFIB專利心房顫動偵測技術
https://www.microlife.com.tw/wp-content/uploads/2020/09/BP3MS1-4K-2-.png
800
800
admin
/wp-content/uploads/2020/09/logo_microlife-3.svg
admin 2020-09-27 16:14:50 2022-05-24 14:37:45 BP 3MS1-4K
https://www.microlife.com.tw/wp-content/uploads/2022/09/BP3BU1-3_800.png
800
800
admin
/wp-content/uploads/2020/09/logo_microlife-3.svg
admin 2020-09-27 16:07:28 2022-09-22 10:23:44 BP 3BU1-3
https://www.microlife.com.tw/wp-content/uploads/2022/09/BP3BJ1-4_800.png
800
800
admin
/wp-content/uploads/2020/09/logo_microlife-3.svg
admin 2020-09-27 15:28:56 2022-09-22 17:15:24 BP 3BJ1-4D 2020 年 9 月 26 日
PAD心跳不規律偵測技術
https://www.microlife.com.tw/wp-content/uploads/2020/09/BPA2Easy-e1607993783246.png
1000
1000
admin
/wp-content/uploads/2020/09/logo_microlife-3.svg
admin 2020-09-26 19:58:25 2022-05-24 15:59:16 BP A2 EASY
https://www.microlife.com.tw/wp-content/uploads/2020/09/BP3BG1-A.png
800
800
admin
/wp-content/uploads/2020/09/logo_microlife-3.svg
admin 2020-09-26 15:16:09 2022-05-26 09:14:04 BP 3BG1-A
https://www.microlife.com.tw/wp-content/uploads/2020/09/BP3AQ1-1_square-1.png
800
800
admin
/wp-content/uploads/2020/09/logo_microlife-3.svg
admin 2020-09-26 15:07:49 2022-05-26 10:01:01 BP 3AQ1-1
https://www.microlife.com.tw/wp-content/uploads/2020/09/BP3AQ1-1.jpg
800
800
admin
/wp-content/uploads/2020/09/logo_microlife-3.svg
admin 2020-09-26 14:52:15 2022-05-26 09:59:47 BP 3AQ1
https://www.microlife.com.tw/wp-content/uploads/2020/09/BP3AR1-3P-2.png
800
800
demo
/wp-content/uploads/2020/09/logo_microlife-3.svg
demo 2020-09-26 14:49:47 2022-05-24 16:02:38 BP 3AR1-3P
https://www.microlife.com.tw/wp-content/uploads/2021/05/BP3ML1-A.png
2400
2400
microlife
/wp-content/uploads/2020/09/logo_microlife-3.svg
microlife 2020-06-10 13:54:25 2022-06-07 09:09:00 BP 3ML1-A
以關懷人類與地球為出發點,貼心設計
2020 年 11 月 21 日
智慧安全蓋式電熱毯
https://www.microlife.com.tw/wp-content/uploads/2020/11/OBP-.png
800
800
admin
/wp-content/uploads/2020/09/logo_microlife-3.svg
admin 2020-11-21 17:49:08 2022-05-24 16:51:21 OBP 2020 年 11 月 21 日
智慧安全蓋式電熱毯
https://www.microlife.com.tw/wp-content/uploads/2020/11/OTD-T.png
800
800
admin
/wp-content/uploads/2020/09/logo_microlife-3.svg
admin 2020-11-21 10:01:41 2022-05-25 10:32:58 OTD -T 2020 年 11 月 21 日
智慧安全蓋式電熱毯
https://www.microlife.com.tw/wp-content/uploads/2020/11/OTQ-T-.png
800
800
admin
/wp-content/uploads/2020/09/logo_microlife-3.svg
admin 2020-11-21 09:08:07 2022-05-25 10:25:43 OTQ -T 2020 年 11 月 20 日
舒適型動力式熱敷墊
https://www.microlife.com.tw/wp-content/uploads/2020/11/FH300-.png
800
800
admin
/wp-content/uploads/2020/09/logo_microlife-3.svg
admin 2020-11-20 09:11:29 2022-05-25 10:27:13 FH 300
https://www.microlife.com.tw/wp-content/uploads/2020/11/FH200C-.png
800
800
admin
/wp-content/uploads/2020/09/logo_microlife-3.svg
admin 2020-11-20 09:10:54 2022-05-25 10:31:38 FH 200CH 2020 年 11 月 20 日
舒適型動力式熱敷墊
https://www.microlife.com.tw/wp-content/uploads/2020/11/FH320-1-.png
800
800
admin
/wp-content/uploads/2020/09/logo_microlife-3.svg
admin 2020-11-20 09:10:27 2022-05-25 10:36:49 FH 320 2020 年 11 月 20 日
舒適型動力式熱敷墊
https://www.microlife.com.tw/wp-content/uploads/2020/11/FH96-1-.png
800
800
admin
/wp-content/uploads/2020/09/logo_microlife-3.svg
admin 2020-11-20 09:09:58 2022-05-25 10:39:04 FH 96 2020 年 11 月 20 日
舒適型乾濕兩用熱敷墊
https://www.microlife.com.tw/wp-content/uploads/2020/11/FH90-.png
800
800
admin
/wp-content/uploads/2020/09/logo_microlife-3.svg
admin 2020-11-20 09:09:01 2022-05-25 10:40:30 FH 90-1 2020 年 11 月 21 日
智慧安全蓋式電熱毯
https://www.microlife.com.tw/wp-content/uploads/2020/11/OBP-.png
800
800
admin
/wp-content/uploads/2020/09/logo_microlife-3.svg
admin 2020-11-21 17:49:08 2022-05-24 16:51:21 OBP 2020 年 11 月 21 日
智慧安全蓋式電熱毯
https://www.microlife.com.tw/wp-content/uploads/2020/11/OTD-T.png
800
800
admin
/wp-content/uploads/2020/09/logo_microlife-3.svg
admin 2020-11-21 10:01:41 2022-05-25 10:32:58 OTD -T 2020 年 11 月 21 日
智慧安全蓋式電熱毯
https://www.microlife.com.tw/wp-content/uploads/2020/11/OTQ-T-.png
800
800
admin
/wp-content/uploads/2020/09/logo_microlife-3.svg
admin 2020-11-21 09:08:07 2022-05-25 10:25:43 OTQ -T 2020 年 11 月 20 日
舒適型動力式熱敷墊
https://www.microlife.com.tw/wp-content/uploads/2020/11/FH300-.png
800
800
admin
/wp-content/uploads/2020/09/logo_microlife-3.svg
admin 2020-11-20 09:11:29 2022-05-25 10:27:13 FH 300
https://www.microlife.com.tw/wp-content/uploads/2020/11/FH200C-.png
800
800
admin
/wp-content/uploads/2020/09/logo_microlife-3.svg
admin 2020-11-20 09:10:54 2022-05-25 10:31:38 FH 200CH 2020 年 11 月 20 日
舒適型動力式熱敷墊
https://www.microlife.com.tw/wp-content/uploads/2020/11/FH320-1-.png
800
800
admin
/wp-content/uploads/2020/09/logo_microlife-3.svg
admin 2020-11-20 09:10:27 2022-05-25 10:36:49 FH 320 2020 年 11 月 20 日
舒適型動力式熱敷墊
https://www.microlife.com.tw/wp-content/uploads/2020/11/FH96-1-.png
800
800
admin
/wp-content/uploads/2020/09/logo_microlife-3.svg
admin 2020-11-20 09:09:58 2022-05-25 10:39:04 FH 96 2020 年 11 月 20 日
舒適型乾濕兩用熱敷墊
https://www.microlife.com.tw/wp-content/uploads/2020/11/FH90-.png
800
800
admin
/wp-content/uploads/2020/09/logo_microlife-3.svg
admin 2020-11-20 09:09:01 2022-05-25 10:40:30 FH 90-1
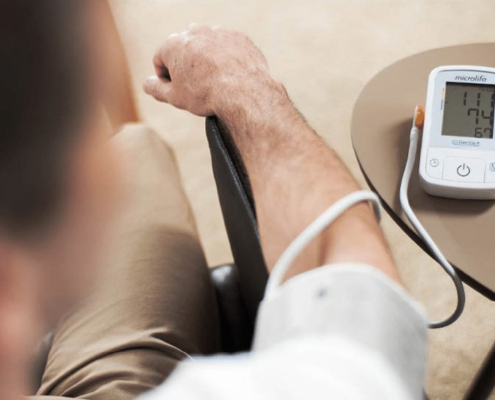
Microlife 的服務據點遍布各地,提供給您最便利的服務。
請依據您的需求,找離您最近的商店!
找不到您要的產品嗎?歡迎直接與我們聯繫,
我們將會派專人回覆您。
Scroll to top
官方網站設計
, CIS企業識別設計
, 設計LOGO
, 設計品牌
, 名片設計
, 網頁設計
, 品牌設計
, 網站設計
, 官網設計
, 響應式網頁設計
, 響應式網站設計
, 公司網頁設計
, 網頁設計
, 教會網站設計設計
, 婚攝
, 婚禮攝影
, 婚紗攝影
, 婚攝推薦
, 美式婚禮攝影
, 美式婚紗攝影
, 孕婦寫真
, 親子寫真
, 家庭寫真
, 美式風格婚紗攝影
, 美式風格婚禮攝影
, 台北美式婚禮攝影推薦
, 血壓量測
, 腕式血壓計
, 全自動手臂式血壓計
, 心房顫動偵測
, 體溫計
, 耳溫槍
, 額溫槍
, 熱敷墊
, 電毯
, 中風
, 高血壓
, 血壓計
, 血壓計推薦
, 血壓計品牌
, 歐姆龍
, 百靈
, 台北攝影棚出租
, 台北攝影棚租借
, 攝影棚出租
, 攝影棚租借
, 攝影場地租借
, 專業形象照
, 形象照拍攝
, 個人形象照
, 孕婦禮服出租
, 孕婦禮服租借
, 自助婚紗
, 自助婚紗攝影
, 台北攝影棚出租推薦
, 台北專業形象照推薦
, 孕婦禮服出租推薦
, 自助婚紗推薦
, 婚攝
, 婚禮攝影
, 婚禮紀錄
, 台北親子寫真
, 台北兒童寫真
, 台北家庭寫真
, 台南親子寫真
, 台中親子寫真
, 高雄親子寫真
, 台南兒童寫真
, 台中兒童寫真
, 高雄兒童寫真
, 台南親子寫真推薦
, 台中親子寫真推薦
, 高雄親子寫真推薦
, 台南家庭寫真
, 台中家庭寫真
, 高雄家庭寫真
, 台南全家福
, 台中全家福
, 高雄全家福
, 婚攝
, 台南婚攝
, 婚禮攝影
, 自助婚紗
, 台南婚禮攝影
, 台南婚禮攝影推薦
, 新秘
, 新娘秘書
, 新娘造型
, 高雄新秘推薦
, 台北新秘推薦
, 新秘Yuki
, 白色夢幻新秘Yuki
, 新娘秘書Yuki
, 新娘助理
, 高雄新秘
, 台北新秘
, 婚禮婚紗造型
, 新娘妝髮造型
, 新秘彩妝造型
, 自助婚紗造型
, 台北新娘秘書推薦
, 高雄新娘秘書推薦
, 花草風造型
, 自然風格造型
, 室內香氛
, 居家香氛
, 房間香氛
, 空間香氛
, 香氛蠟燭
, 香氛精油
, 居家香精
, 精油香氛
, 居家香氛擴香
, 香氛蠟燭推薦
, 房間香氛推薦
, 香氛品牌推薦
, 嚴選香氛
, 香氛推薦
, 精油推薦
, 香精
, 精油
, 根尖手術
, 根管治療
, 牙齒再植手術
, 顯微根管
, 鈣化根管治療
, 再生性根管治療
, 牙根黑影與膿包治療
, 高雄根管治療
, 高雄牙齒醫師
, 高雄顯微根管醫師
, 美白牙齒
, 婚禮顧問
, 婚顧
, 婚禮企劃
, 婚禮主持
, 雙語婚禮主持
, 英文婚禮主持
, Wedding mc
, Wedding Planner
, Bilingual Wedding
, Wedding mc in Taiwan
, Wedding mc in Taipei
, Wedding Planner in Taiwan
, Wedding Planner in Taipei
, Bilingual Wedding mc in Taiwan
, Bilingual Wedding mc in Taipei
, 婚禮顧問推薦
, 婚禮企劃推薦
, 壯陽藥
, 威而鋼
, 犀利士
, 壯陽藥
, 威而鋼
, 犀利士
, 武倍對策
, 我弟很猛
, 禮儀公司
, 葬儀社
, 生命禮儀
, 禮儀服務
, 殯葬服務
, 殯葬禮儀
, 屏東禮儀公司
, 屏東禮儀社
, 屏東葬儀社
, 屏東生命禮儀
, 屏東禮儀服務
, 屏東殯葬服務
, 高雄禮儀公司
, 高雄葬儀社
, 高雄生命禮儀
, 高雄禮儀服務
, 高雄殯葬服務
, 中式禮儀服務
, 西式禮儀服務
, 情趣用品
, 情趣娃娃
, 按摩棒
, 自慰套
, 情趣玩具
, 自慰棒
, 寵物氧氣機
, PetO2 寵物氧氣機
, 寵物製氧機
, 寵物專用氧氣機
, 狗用氧氣機
, 貓用氧氣機
, 犬貓氧氣機
, 寵物魚油
, 犬貓魚油
, 貓用魚油
, 犬用魚油
, 狗魚油
, 貓魚油
, 寵物益生菌
, 犬貓益生菌
, 貓用益生菌
, 犬用益生菌
, 狗狗益生菌
, 貓咪益生菌
, 工業廠房
, 投資辦公室
, 工業地
, 徵收土地
, 土地整治
, 土地開發
, 工業地產投資
, 廠辦整合規劃
 https://www.microlife.com.tw/wp-content/uploads/2023/02/21.jpg
548
800
microlife
/wp-content/uploads/2020/09/logo_microlife-3.svg
microlife2022-04-04 11:00:262023-02-08 13:17:12簡單措施,平穩血壓
https://www.microlife.com.tw/wp-content/uploads/2023/02/21.jpg
548
800
microlife
/wp-content/uploads/2020/09/logo_microlife-3.svg
microlife2022-04-04 11:00:262023-02-08 13:17:12簡單措施,平穩血壓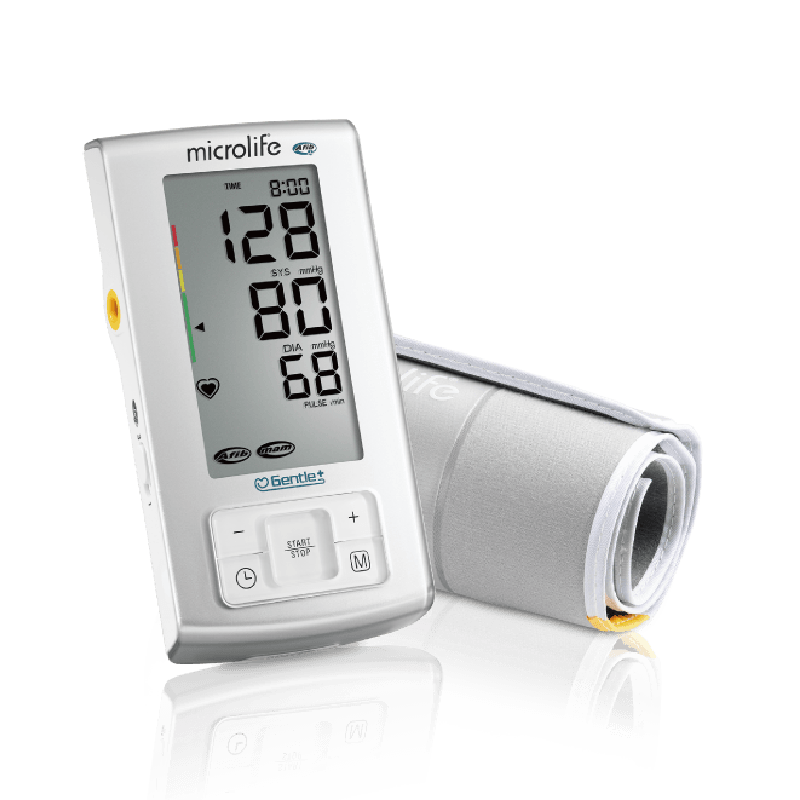




 https://www.microlife.com.tw/wp-content/uploads/2023/02/21.jpg
548
800
microlife
/wp-content/uploads/2020/09/logo_microlife-3.svg
microlife2022-04-04 11:00:262023-02-08 13:17:12簡單措施,平穩血壓
https://www.microlife.com.tw/wp-content/uploads/2023/02/21.jpg
548
800
microlife
/wp-content/uploads/2020/09/logo_microlife-3.svg
microlife2022-04-04 11:00:262023-02-08 13:17:12簡單措施,平穩血壓 https://www.microlife.com.tw/wp-content/uploads/2023/02/2.jpg
548
800
microlife
/wp-content/uploads/2020/09/logo_microlife-3.svg
microlife2022-04-04 10:00:082023-02-08 13:17:31如何正確量測血壓
https://www.microlife.com.tw/wp-content/uploads/2023/02/2.jpg
548
800
microlife
/wp-content/uploads/2020/09/logo_microlife-3.svg
microlife2022-04-04 10:00:082023-02-08 13:17:31如何正確量測血壓 https://www.microlife.com.tw/wp-content/uploads/2023/02/23.jpg
548
800
microlife
/wp-content/uploads/2020/09/logo_microlife-3.svg
microlife2022-04-04 09:00:012023-02-08 13:17:55妊娠、子癇前症(妊娠毒血)的血壓監測
https://www.microlife.com.tw/wp-content/uploads/2023/02/23.jpg
548
800
microlife
/wp-content/uploads/2020/09/logo_microlife-3.svg
microlife2022-04-04 09:00:012023-02-08 13:17:55妊娠、子癇前症(妊娠毒血)的血壓監測 https://www.microlife.com.tw/wp-content/uploads/2023/02/7.jpg
548
800
microlife
/wp-content/uploads/2020/09/logo_microlife-3.svg
microlife2022-04-03 11:00:402023-02-08 13:23:18關於百略耳溫槍的11件事
https://www.microlife.com.tw/wp-content/uploads/2023/02/7.jpg
548
800
microlife
/wp-content/uploads/2020/09/logo_microlife-3.svg
microlife2022-04-03 11:00:402023-02-08 13:23:18關於百略耳溫槍的11件事 https://www.microlife.com.tw/wp-content/uploads/2023/02/16.jpg
548
800
microlife
/wp-content/uploads/2020/09/logo_microlife-3.svg
microlife2022-04-03 10:00:222023-02-08 13:23:35關於體溫管理,你應該知道
https://www.microlife.com.tw/wp-content/uploads/2023/02/16.jpg
548
800
microlife
/wp-content/uploads/2020/09/logo_microlife-3.svg
microlife2022-04-03 10:00:222023-02-08 13:23:35關於體溫管理,你應該知道
 https://www.microlife.com.tw/wp-content/uploads/2023/02/13.jpg
548
800
microlife
/wp-content/uploads/2020/09/logo_microlife-3.svg
microlife2022-04-02 11:00:472023-02-08 13:52:33熱敷舒緩的常見問題
https://www.microlife.com.tw/wp-content/uploads/2023/02/13.jpg
548
800
microlife
/wp-content/uploads/2020/09/logo_microlife-3.svg
microlife2022-04-02 11:00:472023-02-08 13:52:33熱敷舒緩的常見問題 https://www.microlife.com.tw/wp-content/uploads/2023/02/5.jpg
548
800
microlife
/wp-content/uploads/2020/09/logo_microlife-3.svg
microlife2022-04-02 10:00:362023-02-08 13:53:05熱療的好處
https://www.microlife.com.tw/wp-content/uploads/2023/02/5.jpg
548
800
microlife
/wp-content/uploads/2020/09/logo_microlife-3.svg
microlife2022-04-02 10:00:362023-02-08 13:53:05熱療的好處 https://www.microlife.com.tw/wp-content/uploads/2023/02/1.jpg
548
800
microlife
/wp-content/uploads/2020/09/logo_microlife-3.svg
microlife2022-04-02 09:00:422023-02-08 13:53:29熱敷可舒緩痠痛
https://www.microlife.com.tw/wp-content/uploads/2023/02/1.jpg
548
800
microlife
/wp-content/uploads/2020/09/logo_microlife-3.svg
microlife2022-04-02 09:00:422023-02-08 13:53:29熱敷可舒緩痠痛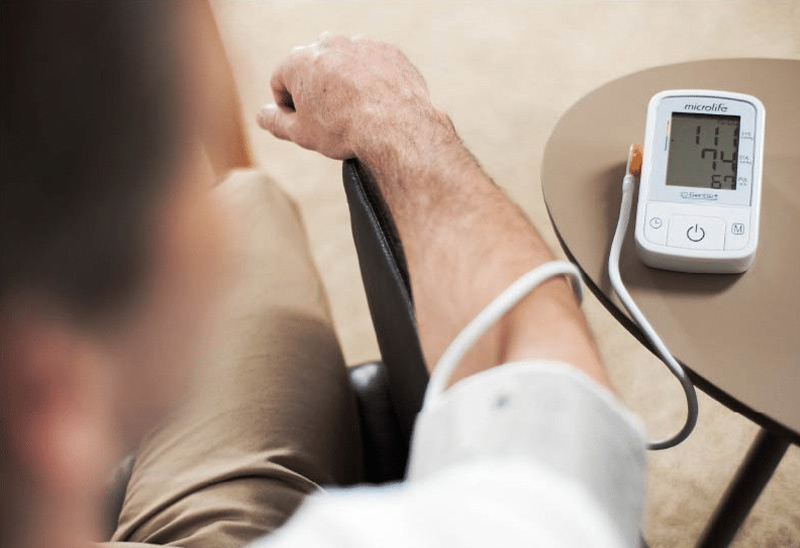 https://www.microlife.com.tw/wp-content/uploads/2023/02/21.jpg
548
800
microlife
/wp-content/uploads/2020/09/logo_microlife-3.svg
microlife2022-04-04 11:00:262023-02-08 13:17:12簡單措施,平穩血壓
https://www.microlife.com.tw/wp-content/uploads/2023/02/21.jpg
548
800
microlife
/wp-content/uploads/2020/09/logo_microlife-3.svg
microlife2022-04-04 11:00:262023-02-08 13:17:12簡單措施,平穩血壓 https://www.microlife.com.tw/wp-content/uploads/2023/02/2.jpg
548
800
microlife
/wp-content/uploads/2020/09/logo_microlife-3.svg
microlife2022-04-04 10:00:082023-02-08 13:17:31如何正確量測血壓
https://www.microlife.com.tw/wp-content/uploads/2023/02/2.jpg
548
800
microlife
/wp-content/uploads/2020/09/logo_microlife-3.svg
microlife2022-04-04 10:00:082023-02-08 13:17:31如何正確量測血壓 https://www.microlife.com.tw/wp-content/uploads/2023/02/23.jpg
548
800
microlife
/wp-content/uploads/2020/09/logo_microlife-3.svg
microlife2022-04-04 09:00:012023-02-08 13:17:55妊娠、子癇前症(妊娠毒血)的血壓監測
https://www.microlife.com.tw/wp-content/uploads/2023/02/23.jpg
548
800
microlife
/wp-content/uploads/2020/09/logo_microlife-3.svg
microlife2022-04-04 09:00:012023-02-08 13:17:55妊娠、子癇前症(妊娠毒血)的血壓監測 https://www.microlife.com.tw/wp-content/uploads/2023/02/7.jpg
548
800
microlife
/wp-content/uploads/2020/09/logo_microlife-3.svg
microlife2022-04-03 11:00:402023-02-08 13:23:18關於百略耳溫槍的11件事
https://www.microlife.com.tw/wp-content/uploads/2023/02/7.jpg
548
800
microlife
/wp-content/uploads/2020/09/logo_microlife-3.svg
microlife2022-04-03 11:00:402023-02-08 13:23:18關於百略耳溫槍的11件事 https://www.microlife.com.tw/wp-content/uploads/2023/02/16.jpg
548
800
microlife
/wp-content/uploads/2020/09/logo_microlife-3.svg
microlife2022-04-03 10:00:222023-02-08 13:23:35關於體溫管理,你應該知道
https://www.microlife.com.tw/wp-content/uploads/2023/02/16.jpg
548
800
microlife
/wp-content/uploads/2020/09/logo_microlife-3.svg
microlife2022-04-03 10:00:222023-02-08 13:23:35關於體溫管理,你應該知道
 https://www.microlife.com.tw/wp-content/uploads/2023/02/13.jpg
548
800
microlife
/wp-content/uploads/2020/09/logo_microlife-3.svg
microlife2022-04-02 11:00:472023-02-08 13:52:33熱敷舒緩的常見問題
https://www.microlife.com.tw/wp-content/uploads/2023/02/13.jpg
548
800
microlife
/wp-content/uploads/2020/09/logo_microlife-3.svg
microlife2022-04-02 11:00:472023-02-08 13:52:33熱敷舒緩的常見問題 https://www.microlife.com.tw/wp-content/uploads/2023/02/5.jpg
548
800
microlife
/wp-content/uploads/2020/09/logo_microlife-3.svg
microlife2022-04-02 10:00:362023-02-08 13:53:05熱療的好處
https://www.microlife.com.tw/wp-content/uploads/2023/02/5.jpg
548
800
microlife
/wp-content/uploads/2020/09/logo_microlife-3.svg
microlife2022-04-02 10:00:362023-02-08 13:53:05熱療的好處